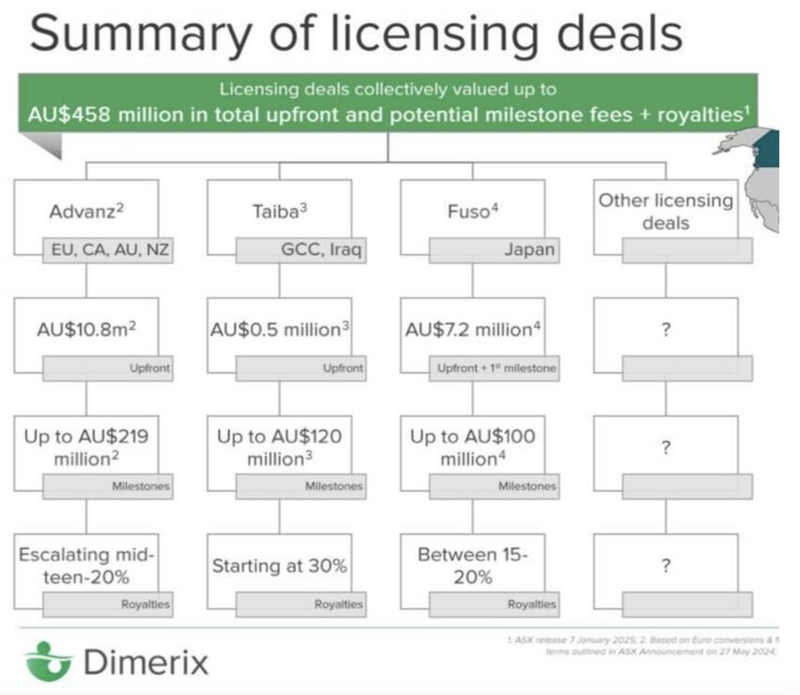
DXB secures $107M licencing deal in Japan, phase 3 results this year.
This week our 2021 Biotech Pick of the Year DXB signed a third licensing deal for its FSGS clinical trial.
FSGS is a rare kidney disease and DXB is just 8 months away from potentially securing accelerated FDA approval to sell this product.
This deal is for the Japan region and worth up to $107M + royalty sales.
There are still two key markets that DXB is yet to find a partner: US and China.
This marks the third licensing deal that DXB has signed, BEFORE the results of its Phase 3 clinical trial have been published, highlighting the “race” for exclusive access to DXB’s treatment.
During the holiday period DXB announced that the crucial 144th patient had been recruited, randomised and dosed - which is the last patient needed to take DXB through to the “interim analysis results”.
We now have a crucial timeframe in August 2025 where DXB will complete its clinical trial and publish the results of the trial shortly afterwards.
The results of this trial will determine if DXB is eligible for conditional or accelerated approval to sell its treatment.
It has been a long 3 years since DXB started the clinical trial, but the company is now just 8 months away from securing real data to show whether its treatment works.
What’s Next for DXB?
✅ Complete recruitment and dosing of 144 patients
As announced on the 30th December last year, this has now been complete.
🔄 Complete recruitment and dosing of 286 patients
Full study recruitment is expected to be complete Q3 2025.
🔄 Second interim results announced
The second interim analysis results will provide us with much more information about the efficacy of the DMX-200 drug. These results will actually show us how effective the treatment is against FSGS using key biomarkers.
These results will be make or break for DXB and should be a huge catalyst for the company.
🔲 Accelerated approvals granted
If the results are successful we expect DXB to move quickly towards accelerated approvals. Even if the results are strong, they will need to be strong enough to warrant accelerated approval of the drug.
🔲 Drug pricing announced for DMX-200
An important factor for the DXB story is what is the drug pricing? This is generally set by negotiations between the payers and providers (think the insurance companies / governments).
Another kidney treatment Sparsentan was priced at US$120,000 per year, per patient.
This gives an idea of what the price would be in the US (the most lucrative market), however other markets would likely command similar high prices to be paid by insurance companies / governments.
🔲 China commercialisation deal
China is a big market for FSGS with over 100,000 potential patients.
🔲 USA commercialisation deal
This is the big one. If DXB is able to sign a commercialisation agreement with the US it will be the most lucrative deal that the company signs.






